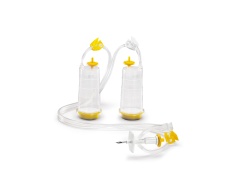
| International pharmacopoeias require the complete sterility of pharmaceutical products that are injected into the blood stream or that otherwise enter the body below the skin surface. As a manufacturer of such products, you are required to supply proof of sterility of the final product batch.Sterisart® NF is a completely closed system for the sterility testing of pharmaceutical products. It is based on the membrane filter method, however it eliminates the procedure of manipulating the filters. By this the main risk of a secondary contamination and false positive results is eliminated. A peristaltic pump transfers the sample into the filtration units, and after rinsing, the filtration units are filled with media and used for incubation of the filters without any contact to the environment. |